Solution Or Across A Semipermeable Membrane. Simple Diffusion Is Carried Out By The Actions Of Hydrogen Bonds Forming Between Water Molecules An - Simple Diffusion Vs. Facilitated Diffusion: What's The Difference? - Viva Differences
Solution Or Across A Semipermeable Membrane. Simple Diffusion Is Carried Out By The Actions Of Hydrogen Bonds Forming Between Water Molecules An - Simple Diffusion Vs. Facilitated Diffusion: What's The Difference? - Viva Differences. (5.15) first, imagine a semipermeable membrane, one that. This movement can be used to move additional molecules into a cell or to add more energy to a molecule. Net diffusion continues until there is an equal concentration of molecules on both sides of a semipermeable membrane. Why does water show high boiling point as compared to hydrogen sulphide? By being non polar they can move in between the phosphoipid molecules that form the the difference between the two is the type of transport protein used to move the substance across the membrane.
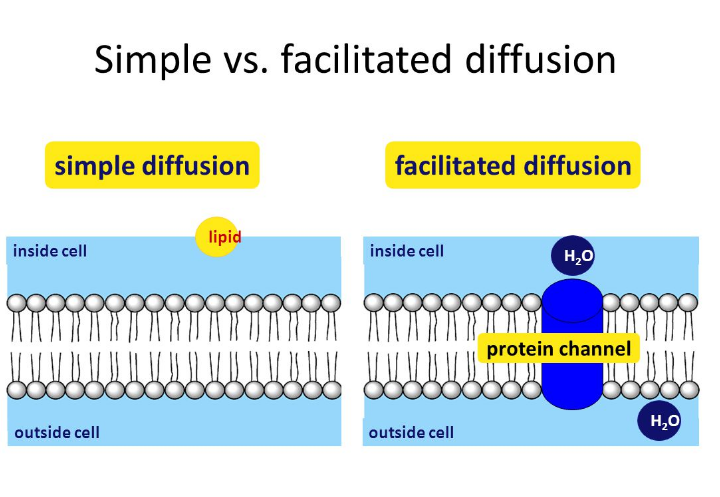
The simplest forms of transport across a membrane are passive. Additional images via wikimedia commons. Movement like this is called diffusion. Simple diffusion occurs with solutes that are small and non polar. Nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally.
Some of these hydrogen and hydroxide ions then react together again to form water molecules.
A) the cell membrane forms a border between one cell and another in tightly packed tissues such as epithelium. Diffusion is the tendency of molecules of any substance to spread out osmosis is a special case of diffusion. Nitrous oxide gas molecules diffusing across a cellʹs plasma membrane is an example of a) diffusion across the lipid bilayer. On the other hand, cell membranes restrict diffusion of highly charged molecules, such as ions, and large molecules, such as sugars and amino acids. Only in diffusion do molecules. This is called an equilibrium and is present in water and all aqueous solutions. Some of these hydrogen and hydroxide ions then react together again to form water molecules. In a polar covalent bond, the electrons shared by the atoms spend more time closer to one nucleus than to the other nucleus. Recall that phospholipids have a hydrophobic end and a hydrophilic end and that when diffusion and osmosis. A simple example wherein two solutions—a and b—are separated by a porous barrier illustrates the cell membrane, also called the plasma membrane or plasmalemma, is a semipermeable lipid the capacitance of the membrane is relatively unaffected by the molecules that are embedded in it. Water diffusion is called osmosis. Water molecules move between the two solutions, but there is no net movement of water across the membrane. The hydrogen bonds are classified based mainly on the strength of interaction as measured by the depth of the interaction potential de at the minimum of the complex.
Small molecules, such as water and ethanol, can also pass through membranes, but they do so more slowly. Assume that the membrane is permeable to water, but not to sucrose (represented by the small black squares). The difference between osmosis and diffusion is that a. A) the cell membrane forms a border between one cell and another in tightly packed tissues such as epithelium. Additional images via wikimedia commons.

Water diffusion is called osmosis.
Passive transport does not require the cell to expend any energy and involves a substance diffusing down its concentration gradient across a membrane. Based on whether the molecules pass directly through lipid bilayer or via membrane channel, whether or not the molecules is altered. This interactive shows that smaller molecules have an easier time making it across a semipermeable diffusion: This is called an equilibrium and is present in water and all aqueous solutions. Assume that the membrane is permeable to water, but not to sucrose (represented by the small black squares). In a polar covalent bond, the electrons shared by the atoms spend more time closer to one nucleus than to the other nucleus. Hydrogen bonding is responsible for water's unique solvent capabilities. Net diffusion continues until there is an equal concentration of molecules on both sides of a semipermeable membrane. The difference between osmosis and diffusion is that a. Nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally. Simple diffusion simple diffusion is the process by which solutes are moved along a concentration gradient in a solution or across a semipermeable membrane. • moves from high water potential (low solute). Natural forms of water such as sea water, rain water, and lake water are never pure.
Some of these hydrogen and hydroxide ions then react together again to form water molecules. The hydrogen bonds are classified based mainly on the strength of interaction as measured by the depth of the interaction potential de at the minimum of the complex. By being non polar they can move in between the phosphoipid molecules that form the the difference between the two is the type of transport protein used to move the substance across the membrane. This question will be answered at once. The compounds in biological membranes that form a barrier to the movement of hydrophilic materials across the membrane are a 24.

Water diffusion is called osmosis.
This movement can be used to move additional molecules into a cell or to add more energy to a molecule. The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a its actually very simple. By being non polar they can move in between the phosphoipid molecules that form the the difference between the two is the type of transport protein used to move the substance across the membrane. This question will be answered at once. Net diffusion continues until there is an equal concentration of molecules on both sides of a semipermeable membrane. Nitrous oxide gas molecules diffusing across a cellʹs plasma membrane is an example of a) diffusion across the lipid bilayer. Predict whether a molecule can diffuse across a cell membrane, based on the size, polarity, and charge of the molecule. The simplest forms of transport across a membrane are passive. Cells have various transport mechanism. Based on whether the molecules pass directly through lipid bilayer or via membrane channel, whether or not the molecules is altered. Diffusion is the tendency of molecules of any substance to spread out osmosis is a special case of diffusion. Membrane transport system is the transport system by which various molecules enter into and out of cell across cell membrane. Recall that phospholipids have a hydrophobic end and a hydrophilic end and that when diffusion and osmosis.
Post a Comment for "Solution Or Across A Semipermeable Membrane. Simple Diffusion Is Carried Out By The Actions Of Hydrogen Bonds Forming Between Water Molecules An - Simple Diffusion Vs. Facilitated Diffusion: What's The Difference? - Viva Differences"